Atomic Number:
Atomic number (z) is the number of protons in an atom.
Since for a neutral atom, the number of electrons is equal to number of protons, so Atomic Number is also equal to the number of electrons in neutral the atom.
- Therefore, the number of valence electron present in sodium is 1. Hence it has got 7 electrons in its outermost shell. Thus, sodium has only one valence electron. A lithium atom has one outer shell electron, so it’s usual valence is +1, but it can lose the electron and have a valence of -1.
- The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Sodium is 11. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Atomic number = Number of protons = Number of Electrons (for neutral atom)
Antares auto tune live free download mac. Example: The atomic number of Iron (Fe) is 26; Fe atom contains 26 protons and 26 electrons.
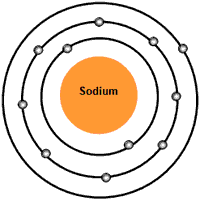
The electrons, which are present in the outermost shell of an atom are called valence electrons. Example: Sodium Atomic number of sodium is 11. Electronic configuration of sodium is 2, 8, 1. In sodium 3 rd shell is the outermost shell (valence shell). In this shell it has 1 electron. Hence the number of valence electrons present in sodium is 1. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The chemical symbol for Sodium is Na. Sodium is a soft, silvery-white, highly reactive metal. Sodium has one valence electron. Valence electrons are electrons found in the outermost shell of an atom. The shell number representing the valence shell will differ depending on the atom in question. For sodium, which is in the 3rd row of the periodic table, the valence electrons will be found in the 3rd shell.
Mass Number:
The mass number (A) is defined as the sum of the number of protons and neutrons present in the nucleus of an atom. It is also referred as Atomic Mass.
Example: Mass number of Nitrogen atom is 14 then it contains 7 protons and 7 neutrons.
Mass number = Number of Protons + Number of Neutrons
Notation of element :
Atomic number Element Mass number= Z XA
Example: Sodium atom has notation as 11Na23 .
Question: An atom X has mass number 40 and atomic number 18. Find out the number of protons, number of electrons and number of neutrons present in the atom X?
Solution:
Number of protons:
Number of protons = Atomic number = 18
Number of electrons:
In an atom number of electrons = Number of protons = 18
Number of Neutrons = Mass number – Atomic number = 40-18 = 22
Electronic Configuration :
The arrangement of electrons in the shells is known as electronic configuration.
Electrons are present in fixed energy levels called Shells. These Shells are also called Orbits. These orbits or Shells are represented by the letters K, L, M, N,… or the numbers n = 1, 2, 3, 4,5,.….
Note: Shell and Orbits are not identical ; They are different ideas. Here for simplification, Students are advised to consider the sense same. Shell more correctly indicates a Sphere (a three dimensional object) while Orbits are Circular paths (A two dimensional object). If Bohr’s Model is considered to be valid; Shells and Orbits are similar for indication purpose.

Rules for accommodating electrons in various shells (Bohr-Bury Rules)
- The maximum number of electrons that can be accommodated in any energy level of the atom is given by a formula 2n2 where n is the number of that Orbit number/ Shell Number / Energy level.
| Orbit Number | Shell Name | Maximum number of Electrons (Using 2n2) |
| 1 | K Shell | 2 |
| 2 | L Shell | 8 |
| 3 | M Shell | 18 |
| 4 | N Shell | 32 |
2. After a series of experiments and a detailed study by scientists like Louis de Broglie, Schrödinger, Somerfield and others proved that shells or energy levels have Sub Shells within them. Electrons are present in these sub shells which constitute a Shell.
Types of Sub Shell : Sub shells are s, p, d and f types.
Every sub-shell can accommodate a fixed number of electrons.
“s” sub shell can hold a maximum of 2 electrons.
“p” sub shell can hold a maximum of 6 electrons.
“d” sub shell can hold a maximum of 10 electrons.
“f” sub shell can hold a maximum of 14 electrons.
Arrangements of Shell and Sub – Shells :
| Shell Name | Sub – Shell name | Number of Electrons in that sub shell |
| K shell (1st Shell) | 1s | 2 |
| L Shell (2nd Shell) | 2s 2p | 2 6 |
| M Shell (3rd Shell) | 3s 3p 3d | 2 6 10 |
Energy Order of different Sub-Shells :
1s<2s<2p<3s<3p<4s<3d<4p<5s……
3. Electrons are filled up in an atom from Lower Energy Level to Higher Energy Level. This rule is called Aufbau Principle.
Electronic Configuration of Sodium atom:
Sodium has atomic number 11 and mass number 23.
The nucleus of sodium has 11 protons and 12 neutrons and it is surrounded by 11 electrons.
Now Its electronic configuration is 1s2, 2s2, 2p6, 3s1
| Note: you can write electronic configuration easily, follow steps : 1. Write the sub-shell energy sequence: 1s<2s<2p<3s<3p<4s<3d<4p<5s 2. Check how many electrons are present in the atom 3. Fill these electrons in the sequence till all electrons are not exhausted; remember sub-shell can accommodate maximum number of electrons as s sub-shell = 2 ; p sub-shell =6, d sub-shell = 10 |
The electronic configuration of sodium: 1s2, 2s2, 2p6, 3s1 can also be written as 2, 8, 1 (indicating 1st shell has 2 electrons, 2nd shell has 8 electrons, 3rd shell has 1 electron).
K shell or I shell = 2 electrons
L– Shell or II shell = 8 electrons
M– Shell or III shell = 1 electron
∴ Electronic configuration of sodium atom = 2, 8, 1
Similarly the electronic configuration of other atoms are :
| Element | Atomic Number | Electronic Configuration Type 1 | Electronic Configuration Type 2 |
| Hydrogen (H) | 1 | 1s1 | 1 |
| Lithium (Li) | 3 | 1s2, 2s1 | 2,1 |
| Carbon (C) | 6 | 1s2, 2s2, 2p2 | 2,4 |
| Aluminium (Al) | 13 | 1s2, 2s2, 2p6,3s2 ,3p1 | 2,8,3 |
| Scandium (Sc) | 21 | 1s2, 2s2, 2p6, 3s2, 3p6,4s2,3d1 | 2,8,9,2 |
| Vanadium (V) | 23 | 1s2, 2s2, 2p6, 3s2, 3p6,4s2,3d3 | 2,8,11,2 |
| Iron (Fe) | 26 | 1s2, 2s2, 2p6, 3s2, 3p6,4s2,3d6 | 2,8,14,2 |
Note the changes in the electronic configuration of Sc, V, Fe .
Q. Now try to write the electronic configuration of
(a) Boron (B) atomic number = 5
(b) Florine (F) atomic number = 9
(c) Titanium (Ti) atomic number = 22
(d) Nickel (Ni) atomic number = 28
Valence electrons:
Number Of Valence Electrons In Sodium Na
The electrons, which are present in the outermost shell of an atom are called valence electrons.
Example: Sodium
Atomic number of sodium is 11
Electronic configuration of sodium is 2, 8, 1 Best free vector graphics software for mac.
In sodium 3rd shell is the outermost shell (valence shell). In this shell it has 1 electron. Hence the number of valence electrons present in sodium is 1.
The chemical properties of an atom are dependent on these valence electrons. Since they are ones that are participate in a chemical reaction.
- Elements having valence electrons 1, 2 or 3 are called metals.
Exception: Hydrogen has 1 valence electron but it is not consider as a metal.
- These elements lose electrons easily and form a positively charged ion called cation.
Example: Na – e– → Na+
- Elements having valence electrons 4, 5, 6 or 7 are called as Non-metals
- These elements gain electrons and forms a negatively charged ion called anion.
Example: F + e– → F–
- Elements with same number of valence electrons have similar chemical properties; whereas the elements with different valence electrons have different chemical properties.
Valency:
The combining capacity of the atoms to form molecules either with same or different elements is defined as Valency.
Valency can be considered as number of Valence electrons; but in some cases it may have different meaning . It is due to the combining property of atoms to form stable molecules.
The valency of element is either equal to the number of valency electron is it atom or equal to number of electron required to complete to octet (eight electrons) in valency shell.
For example :
- Atom contains less than four electrons in its outermost shell, the Valency of an atom is equal to the number of electrons present in the valence shell. More correctly it is called Electrovalency.
Example: Sodium has one electron in its outermost shell, so the Valency of sodium is 1.
Calcium has two electrons in its outermost shell, so the valency of calcium is 2.
Aluminum has three electrons in its outermost shell, so the valency of aluminum is 3.
- If the outer shell has more than four electrons, the valency = 8 – the number of electrons in the outer shell. More correctly it is called Covalency.
- Covalency :Number of electrons shared by one atom of an element to achieve inert gas configuration.
For Chlorine (atomic number = 17) electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p5 or 2,8,7.
Its outermost shell has 7 electrons , therefore its valency can be either 7 or 8-7=1.
1 valency indicates Cl combining capacity is 1 or it can form 1 chemical bond in its molecules.
Octet Rule:
An element with 8 electrons in an outermost-shell is said to possess a complete Octet.
Atoms or ions with octet configuration are stable.
Example: Noble gases possess complete Octet configuration ; they are nonreactive and stable (Except Helium, which possess 2 electrons in its outermost shell).
Atomic mass: The total number of protons and neutrons present in one atom of an element is called atomic mass.
Helium has two protons and two neutrons.
Mass number of He = 2 + 2 = 4
Mass of He atom 2 + 2 = 4u (unified mass)
Isotopes: Atoms of the same element with the same atomic number but different atomic masses are called Isotopes.
Examples: 1H1, 1H2, 1H3 are the isotopes of hydrogen,6 C12 ; 6C14 are isotopes of carbon.
| The chemical properties of all the isotopes of an element are the same. This is due to the presence of same number of electrons. |
Isobars: Atoms of different elements with different atomic numbers but have the same mass number are called Isobars.
Example: 18 Ar40, 19 K40 and 20Ca40.
Reasons for chemical reactivity of an atom: The chemical activity of an atom depends on the number valence electrons. An atom with complete octet configuration is chemically inert and it does not participate in chemical reactions.
Example: Noble gases.
The atoms of element with incomplete octet configuration are chemically active. These elements combine with others to attain stable electronic configuration (octet configuration).
In simple words, atoms combine together so that they acquire 8 electrons in their outermost shell or valence shell.
Example 1: Hydrogen has one electron and it requires one more electron to attain the nearest inert gas configuration. To achieve this each hydrogen atom contributes an electron and form hydrogen molecule.
Example 2: Chlorine atom has 7 electrons in its valence shell and it requires 1 more electron to attain the stable octet configuration. To achieve this, each chlorine atom shares an electron with each other and form chlorine molecule.
Learning Objectives
- To use Lewis electron dot symbols to predict the number of bonds an element will form.
Why are some substances chemically bonded molecules and others are an association of ions? The answer to this question depends upon the electronic structures of the atoms and nature of the chemical forces within the compounds. Although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: ionic bonds, covalent bonds, and metallic bonds. In this chapter, each type of bond wil be discussed and the general properties found in typical substances in which the bond type occurs
- Ionic bonds results from electrostatic forces that exist between ions of opposite charge. These bonds typically involves a metal with a nonmetal
- Covalent bonds result from the sharing of electrons between two atoms. The bonds typically involves one nonmetallic element with another
- Metallic bonds These bonds are found in solid metals (copper, iron, aluminum) with each metal bonded to several neighboring groups and bonding electrons free to move throughout the 3-dimensional structure.
Each bond classification is discussed in detail in subsequent sections of the chapter. Let's look at the preferred arrangements of electrons in atoms when they form chemical compounds.
Lewis Symbols
At the beginning of the 20th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis dot symbols) that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis dot symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons.
Lewis Dot symbols:
- convenient representation of valence electrons
- allows you to keep track of valence electrons during bond formation
- consists of the chemical symbol for the element plus a dot for each valence electron
To write an element’s Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol. Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). The next dots, for elements with more than four valence electrons, are again distributed one at a time, each paired with one of the first four. For example, the electron configuration for atomic sulfur is [Ne]3s23p4, thus there are six valence electrons. Its Lewis symbol would therefore be:
Fluorine, for example, with the electron configuration [He]2s22p5, has seven valence electrons, so its Lewis dot symbol is constructed as follows:
Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Consider the symbol for nitrogen in Figure 8.1.2. The Lewis dot symbol explains why nitrogen, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. Boron, which also has three unpaired valence electrons in its Lewis dot symbol, also tends to form compounds with three bonds, whereas carbon, with four unpaired valence electrons in its Lewis dot symbol, tends to share all of its unpaired valence electrons by forming compounds in which it has four bonds.
The Octet Rule
In 1904, Richard Abegg formulated what is now known as Abegg's rule, which states that the difference between the maximum positive and negative valences of an element is frequently eight. This rule was used later in 1916 when Gilbert N. Lewis formulated the 'octet rule' in his cubical atom theory. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. Atoms will react to get in the most stable state possible. A complete octet is very stable because all orbitals will be full. Atoms with greater stability have less energy, so a reaction that increases the stability of the atoms will release energy in the form of heat or light ;reactions that decrease stability must absorb energy, getting colder.
When discussing the octet rule, we do not consider d or f electrons. Only the s and p electrons are involved in the octet rule, making it a useful rule for the main group elements (elements not in the transition metal or inner-transition metal blocks); an octet in these atoms corresponds to an electron configurations ending with s2p6.
Definition: Octet Rule
A stable arrangement is attended when the atom is surrounded by eight electrons. This octet can be made up by own electrons and some electrons which are shared. Thus, an atom continues to form bonds until an octet of electrons is made. This is known as octet rule by Lewis.
- Normally two electrons pairs up and forms a bond, e.g., (ce{H_2})
- For most atoms there will be a maximum of eight electrons in the valence shell (octet structure), e.g., (ce{CH_4})
The noble gases rarely form compounds. They have the most stable configuration (full octet, no charge), so they have no reason to react and change their configuration. All other elements attempt to gain, lose, or share electrons to achieve a noble gas configuration.
Example (PageIndex{1}): Salt
The formula for table salt is NaCl. It is the result of Na+ ions and Cl- ions bonding together. If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. In the process, a great amount of light and heat is released. The resulting salt is mostly unreactive — it is stable. It will not undergo any explosive reactions, unlike the sodium and chlorine that it is made of. Why?
Solution
Referring to the octet rule, atoms attempt to get a noble gas electron configuration, which is eight valence electrons. Sodium has one valence electron, so giving it up would result in the same electron configuration as neon. Chlorine has seven valence electrons, so if it takes one it will have eight (an octet). Chlorine has the electron configuration of argon when it gains an electron.
The octet rule could have been satisfied if chlorine gave up all seven of its valence electrons and sodium took them. In that case, both would have the electron configurations of noble gasses, with a full valence shell. However, their charges would be much higher. It would be Na7- and Cl7+, which is much less stable than Na+ and Cl-. Atoms are more stable when they have no charge, or a small charge.
Lewis dot symbols can also be used to represent the ions in ionic compounds. The reaction of cesium with fluorine, for example, to produce the ionic compound CsF can be written as follows:
No dots are shown on Cs+ in the product because cesium has lost its single valence electron to fluorine. The transfer of this electron produces the Cs+ ion, which has the valence electron configuration of Xe, and the F− ion, which has a total of eight valence electrons (an octet) and the Ne electron configuration. This description is consistent with the statement that among the main group elements, ions in simple binary ionic compounds generally have the electron configurations of the nearest noble gas. The charge of each ion is written in the product, and the anion and its electrons are enclosed in brackets. This notation emphasizes that the ions are associated electrostatically; no electrons are shared between the two elements.
Atoms often gain, lose, or share electrons to achieve the same number of electrons as the noble gas closest to them in the periodic table.
As you might expect for such a qualitative approach to bonding, there are exceptions to the octet rule, which we describe elsewhere. These include molecules in which one or more atoms contain fewer or more than eight electrons.
Summary
Lewis dot symbols can be used to predict the number of bonds formed by most elements in their compounds. One convenient way to predict the number and basic arrangement of bonds in compounds is by using Lewis electron dot symbols, which consist of the chemical symbol for an element surrounded by dots that represent its valence electrons, grouped into pairs often placed above, below, and to the left and right of the symbol. The structures reflect the fact that the elements in period 2 and beyond tend to gain, lose, or share electrons to reach a total of eight valence electrons in their compounds, the so-called octet rule. Hydrogen, with only two valence electrons, does not obey the octet rule.
Valence Electrons For S
Contributors and Attributions
Mike Blaber (Florida State University)
- National Programme on Technology Enhanced Learning (India)

