- Atomic Mass Of First 20 Elements
- See Full List On Lenntech.com
- Hydrogen
- Relative Atomic Mass Of Elements
- Atomic Mass Of First 20 Elements In Whole Numbers
Science students in high school and colleges often find it a bit difficult to memorize the first 20 elements of the periodic table and this has posed some challenge in school especially during examinations or some kind of academic work that involves the use of any of the 20 elements of the periodic table either in determining their atomic number, mass number or reactivity.
These first 20 elements of the periodic table run across all the 8 groups in the table starting from the group on element down to the group eight elements or what is generally known as the inert gases or rare gases. As students of chemistry, understanding the chemical behavior, properties and reactions of the first twenty elements of the periodic table is the first step in having an authoritative knowledge of chemistry and its applications in all of its branches.
It is very pertinent that every science student at least at the ordinary level stage understand how the elements in the periodic table are groped and arranged in periods and groups and what properties inform the groupings of these elements. I have since learned that importance of the first 20 elements in scientific study and exploits and have devised a methodology of memorizing these first 20 elements without must task to my brain power!
Now here we go…
He(H)
Start studying First 20 Elements - Atomic Mass (Rounded). Learn vocabulary, terms, and more with flashcards, games, and other study tools. Using this method, students can easily write atomic mass of first 20 elements of the periodic table.For more videos on Physics, Chemistry and Mathematics ref. Atomic mass is a characteristic of an atom that is a measure of its size. It is plays a major role in the chemical properties of elements. This article discusses atomic mass and how it is calculated. Further fractionation took place in the formation of the Earth by planetary differentiation: Earth's core, which makes up 31.5% of the mass of the Earth, has approximate composition Fe 25 Ni 2 Co 0.1 S 3; the mantle makes up 68.1% of the Earth's mass and is composed mostly of denser oxides and silicates, an example being olivine, (Mg,Fe) 2 SiO.
Has(He)
Light(Li)
Brain(Be)
But(Bo)
Could(C)
Not(N)
Offer(O)
Full(Fl)
Nine(Ne)
Subjects(Sodium, NA).
Many(Mg)
Art(Al)
Students(Si)
Pose(P)
Some(S)
Complain(Cl)
About(Ar)
Passing(potassium, K)
Chemistry(Ca)
Atomic Mass Of First 20 Elements
Brief history of the periodic table.
Discovery of elements by chemists ushered in another need for studying and learning in Chemistry.
There are about 106 Elements that are known and each element discovered has to be studied.
Elements discovered have wide range of physical and chemical properties. Many are found to behave in similar ways. To make their studies convenient and orderly, chemists began to think about how they could be arranged such that the trend of behavior could be preserved among elements to have a wide range of similar physical and chemical properties.
First 20 Elements of the Periodic Table:
1. Hydrogen – H:
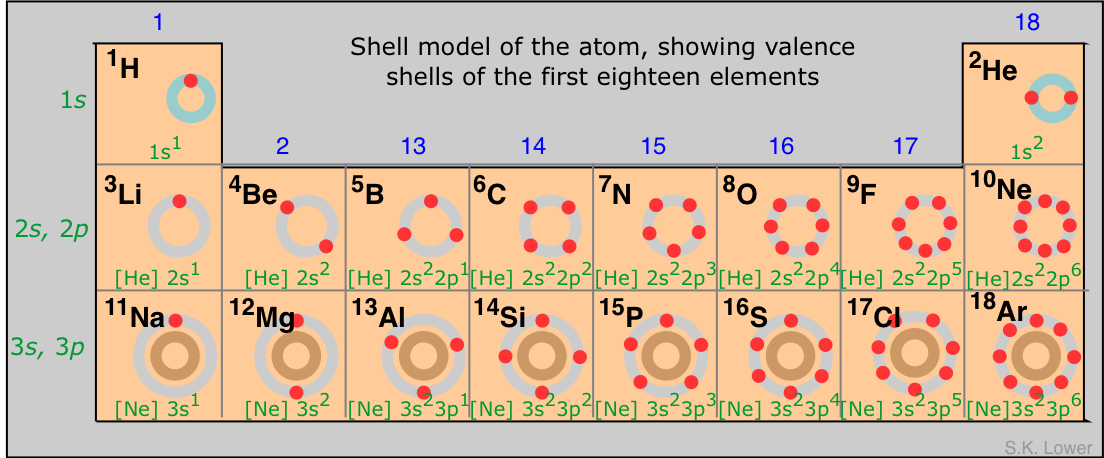
Symbol H. A colorless, odorless gaseous chemical element. It is the lightest and the most abundant element in the universe. It is present in water and in all organic compounds.
2. Helium – He:
Symbol He. A colorless, odorless gaseous non – metallic element belonging to group 18 of the periodic table. Helium has the lowest boiling point of all substances and can be solidified only under pressure.
3. Lithium – Li:
Symbol Li. A soft silvery metal, the first member of group 1 of the periodic table. It is a rare element found in spodumene, petalite, the mica lepidolite and certain brines. It is usually extracted by treatment with sulfuric acid to give sulphate, which is converted to the chloride. This is mixed with a small amount of potassium chloride, melted, and electrolysed.
4. Beryllium – Be:
Symbol Be. A grey metallic element of group 2 of the periodic table. It is used to manufacture Be-Cu alloys, which are used in nuclear reactors as reflectors and moderators because of their low absorption cross section.
5. Boron – B:
Symbol B. An element of the group 13 of the periodic table. It forms two allotropes, amorphous and metallic boron. The metallic form is very hard and is a poor electrical conductor at room temperature.
6. Carbon – C:
Symbol C. A non – metallic element belonging to group 14 of the periodic table. Carbon has three main allotropic forms.
*Diamond: occurs naturally and can be produced synthetically. It is extremely hard and has highly refractive crystals.
7. Nitrogen – N: Adobe acrobat download mac free trial.
Symbol N. A colorless gaseous element belonging to group 15 of the periodic table. It occurs in air (about 78% by volume) and us essential constituent of proteins and nucleic acids in living organisms.
Nitrogen is obtained for industrial purposes by fractional distillation of liquid in air. Pure nitrogen can be obtained in the laboratory by heating a metal azide.
8. Oxygen – O:
Symbol O. A colorless odourless gaseous element belonging to group 16 of the periodic table. It is the most abundant element in the earth’s crust and is present in the atmosphere (28% by volume). Atmospheric oxygen is of vital importance for all organisms that carry out aerobic respiration.
9. Fluorine – F:
Symbol F. A poisonous pale yellow gaseous element belonging to group 17 of the periodic table.
The main mineral sources are *fluorite and *cryolite. The element is obtained by electrolysis of a molten mixture of potassium fluoride and hydrogen fluoride.
10. Neon – Ne:
Symbol Ne. A colorless gaseous element belonging to group 18 of the periodic table.
Neon occurs in air (0.0018% by volume)and is obtained by fractional distillation of liquid air.
11. Sodium – Na:
Symbol Na. A soft silvery reactive element belonging to group 1 of the periodic table. Sodium occurs as the chloride in Sea water and in the mineral halite.
12. Magnesium – Mg:
Symbol Mg. A silvery metallic element belonging to group 2 of the periodic table. The element is found in a number of minerals, including magnesite, dolomite and carnallite.
13. Aluminum – Al:
Symbol Al. A silvery white lustrous metallic element belonging to group 3 of the periodic table. The metal itself nus highly reactive but it’s protected by a thin transparent layer of the oxide, which form quickly in air.
14. Silicon – Si:
Symbol Si. Silicon is a metalloid element belonging to group 14 of the periodic table. Silicon is the second most abundant element in the earth’s crust (25.7% by weight ) occuring in various forms of silicon (IV) oxide (e.g *quartz) and in *silicate minerals.
15. Phosphorus – P:
Symbol P. A non – metallic element belonging to group 25 of the periodic table. It occurs in various phosphate rocks, from which it is extracted by heating with carbon (come) and silicon (IV) oxide in an electric furnace (1500°C).
16. Sulphur – S:
Symbol S. A yellow non – metallic element belonging to group 16 of the periodic table. The element occurs in many sulphide and sulphate minerals.
17. Chlorine – Cl:
Symbol Cl. A halogen element. It is a poisonous greenish-yellow gas and it occurs widely in nature as sodium chloride and in Sea water as halite, carnallite and sylvite.
18. Argon – Ar:
Symbol Ar . A mono atomic Noble gas present in air (0.93%);Argon is separated from liquid air by fractional distillation.
19. Potassium – K:
Symbol K. A soft silvery metallic element belonging to group 1 of the periodic table. The element occurs in Sea water and in a number of minerals, such as sylvite, carnallite and kainite.
20. Calcium – Ca:
Symbol Ca. A soft grey metallic element belonging to group 2 if the periodic table. The element is extracted by electrolysis of fused calcium chloride and is used as a getter in vaccum systems and a deoxidizer in producing non-ferrous alloys.

Since chemistry is somewhat an abstract subject especially when equations and chemical formula are used, it tends to pose some problem for young chemistry students in understanding how to deal with memorizing elements or cramming their chemical reactions and equations especially during examinations or in practical classes. The above simple methodology of memorizing the first 20 elements of the periodic table is a sure step for a beginner to better acquaint him or herself with the rudiments of chemistry and its numerous applications in metallurgy, manufacturing, medicine and pharmacy.
Learning Objective
- Calculate the average atomic mass of an element given its isotopes and their natural abundance
Key Points
- An element can have differing numbers of neutrons in its nucleus, but it always has the same number of protons. The versions of an element with different neutrons have different masses and are called isotopes.
- The average atomic mass for an element is calculated by summing the masses of the element’s isotopes, each multiplied by its natural abundance on Earth.
- When doing any mass calculations involving elements or compounds, always use average atomic mass, which can be found on the periodic table.
Terms
- natural abundanceThe abundance of a particular isotope naturally found on the planet.
- average atomic massThe mass calculated by summing the masses of an element’s isotopes, each multiplied by its natural abundance on Earth.
- mass numberThe total number of protons and neutrons in an atomic nucleus.
The atomic number of an element defines the element’s identity and signifies the number of protons in the nucleus of one atom. For example, the element hydrogen (the lightest element) will always have one proton in its nucleus. The element helium will always have two protons in its nucleus. Clear history on macbook air.
Isotopes
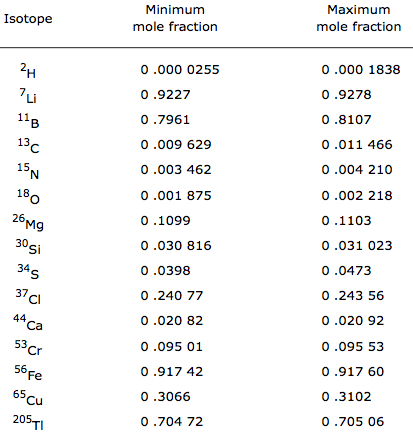
Atoms of the same element can, however, have differing numbers of neutrons in their nucleus. For example, stable helium atoms exist that contain either one or two neutrons, but both atoms have two protons. These different types of helium atoms have different masses (3 or 4 atomic mass units), and they are called isotopes. For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number. This is because each proton and each neutron weigh one atomic mass unit (amu). By adding together the number of protons and neutrons and multiplying by 1 amu, you can calculate the mass of the atom. All elements exist as a collection of isotopes. The word ‘isotope’ comes from the Greek ‘isos’ (meaning ‘same’) and ‘topes’ (meaning ‘place’) because the elements can occupy the same place on the periodic table while being different in subatomic construction.
Calculating Average Atomic Mass
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance (the decimal associated with percent of atoms of that element that are of a given isotope).
Average atomic mass = f1M1 + f2M2 + … + fnMn where f is the fraction representing the natural abundance of the isotope and M is the mass number (weight) of the isotope.
The average atomic mass of an element can be found on the periodic table, typically under the elemental symbol. When data are available regarding the natural abundance of various isotopes of an element, it is simple to calculate the average atomic mass.
- For helium, there is approximately one isotope of Helium-3 for every million isotopes of Helium-4; therefore, the average atomic mass is very close to 4 amu (4.002602 amu).
- Chlorine consists of two major isotopes, one with 18 neutrons (75.77 percent of natural chlorine atoms) and one with 20 neutrons (24.23 percent of natural chlorine atoms). The atomic number of chlorine is 17 (it has 17 protons in its nucleus).
To calculate the average mass, first convert the percentages into fractions (divide them by 100). Then, calculate the mass numbers. The chlorine isotope with 18 neutrons has an abundance of 0.7577 and a mass number of 35 amu. To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
Average atomic mass of chlorine = (0.7577 [latex]cdot[/latex] 35 amu) + (0.2423 [latex]cdot[/latex] 37 amu) = 35.48 amu
Another example is to calculate the atomic mass of boron (B), which has two isotopes: B-10 with 19.9% natural abundance, and B-11 with 80.1% abundance. Therefore,
Average atomic mass of boron = (0.199
[latex]cdot[/latex]
10 amu) + (0.801
[latex]cdot[/latex]
11 amu) = 10.80 amu
Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/mass_number
Wiktionary
CC BY-SA 3.0.
http://www.boundless.com//biology/definition/atomic-mass–2
Boundless Learning
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/natural%20abundance
Wikipedia
CC BY-SA 3.0.
See Full List On Lenntech.com
http://en.wiktionary.org/wiki/isotope
Wiktionary
CC BY-SA 3.0.
http://en.wikibooks.org/wiki/Introductory_Chemistry_Online/Measurements_and_Atomic_Structure
Wikibooks
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Average_atomic_mass
Wikipedia
CC BY-SA 3.0.
Hydrogen
http://en.wikipedia.org/wiki/Atomic_mass
Wikipedia
CC BY-SA 3.0.
Relative Atomic Mass Of Elements
Atomic Mass Of First 20 Elements In Whole Numbers
http://en.wikipedia.org/w/index.php?title=File:Stylised_Lithium_Atom.svg&page=1
Wikipedia
GNU FDL.
